Background Information
What is Desalination?
Desalination refers to any process that reduces the amount of salt in water, usually for the purpose of making it suitable for drinking, irrigation, or industrial use. Because global demands for fresh water are outpacing nature's capacity to replenish our supply, many people are turning to desalination technologies.
The water fed into a desalination system can be seawater (30-35 ppt or 3.0-3.5% salinity) or what is known as "brackish" water which contains less salt than seawater. For example, some well water is too brackish for drinking and must be desalinated. The higher the salinity of the feed water, the more energy is needed to desalinate it.
While desalination offers great promise, the energy requirements can be enormous. Scientists and engineers are working to develop desalination equipment and processes which are energy and cost effective, which can handle the volumes of water we need, and which deal responsibly with both water intake and brine disposal.
LEARN MORE...
The simulation applet on this site models water desalination by electrodialysis. Check out How Does Electrodialysis Work? to find out more!
Simulation Applet
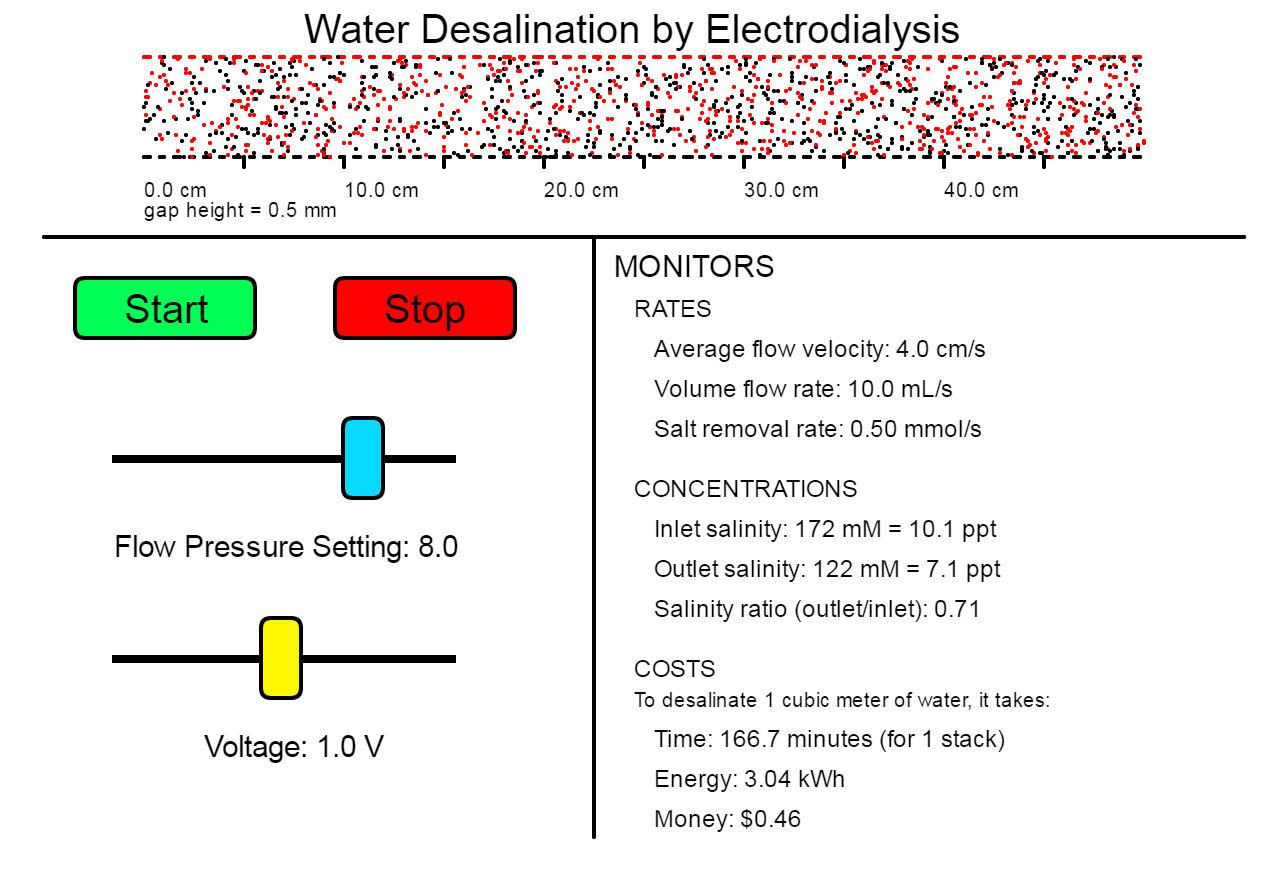
What Can I Do With This Simulation?
BASIC CHALLENGE
In this challenge, you will design a desalination process using the simulation. You will need to decide the dimensions of your cells and cell stack, the flow pressure level, and the amount of voltage to apply.
Your Mission: Design a process to desalinate brackish water with salinity 7 ppt into drinkable water (no more than 1 ppt) for as little cost per volume as you can.
Think you got it? Try Additional Challenges....