Background Information
How Does Electrodialysis Work?
Salt in water takes the form of ions which, because they are charged, move under the influence of an electric field. Electrodialysis uses an applied electric field to remove these salt ions through ion-permeable membranes.
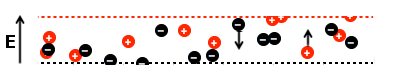
The positive ions (cations) move in one direction while the negative ions (anions) move in the opposite direction. In the diagram, the electric field is oriented so that cations move upward and anions move downward.
The cation-permeable membrane at the top of the channel allows cations to leave, and the anion-permeable membrane at the bottom allows anions to leave. Play the video and watch the cations and anions closely!
What's Going On Here?
In the video, what you see is actually a stack of two "cell pairs," each consisting of a channel for the water that is being desalinated, and a channel where the brine is being concentrated. The number of cell pairs in an actual stack varies depending on the electrodialysis system, with as many as 600 cell pairs in a typical industry-scale system.
Did you notice that the type of ion-permeable membrane alternates? Try watching the third channel from the top (a desalination channel): Cations readily leave out the top, and anions out the bottom. However, at the same time, cations are trying to move upward from below, and anions are trying to move downward from above. But because of the way the membranes are arranged, these ions are blocked from entering. This allows half the channels to get more and more dilute while half the channels get more and more concentrated.
Simulation Applet
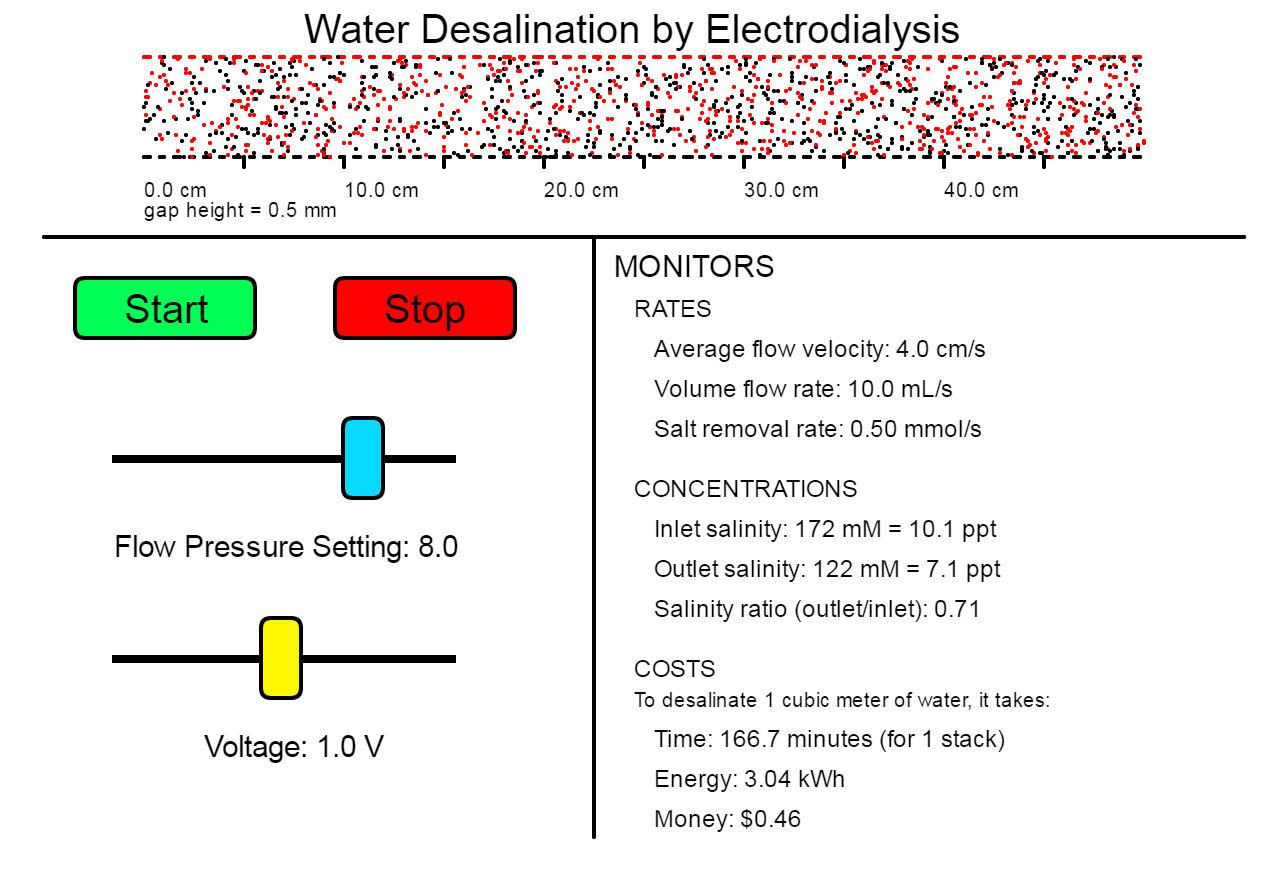
What Can I Do With This Simulation?
BASIC CHALLENGE
In this challenge, you will design a desalination process using the simulation. You will need to decide the dimensions of your cells and cell stack, the flow pressure level, and the amount of voltage to apply.
Your Mission: Design a process to desalinate brackish water with salinity 7 ppt into drinkable water (no more than 1 ppt) for as little cost per volume as you can.
Think you got it? Try Additional Challenges....